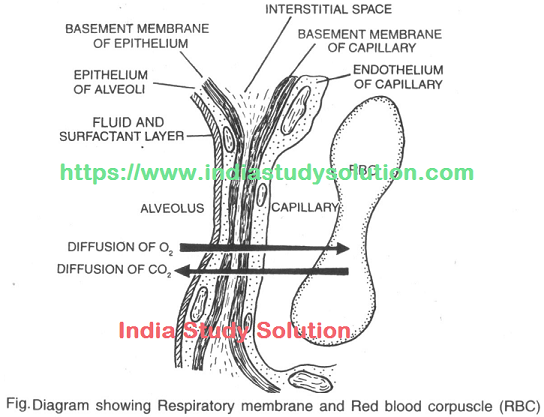
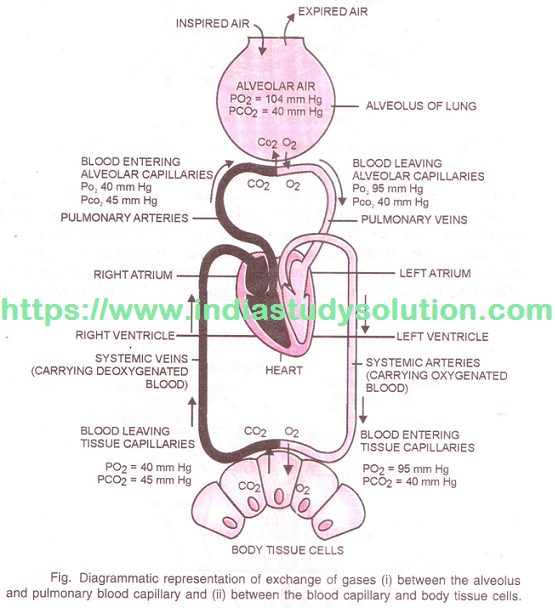
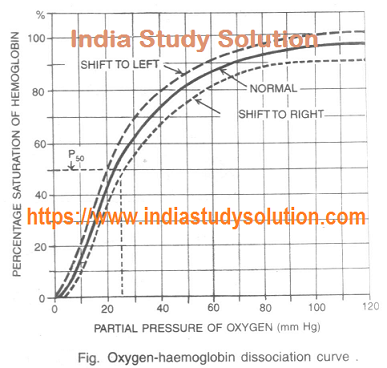
Hi Friends,
You may be aware that this chapter ‘Units, Dimensions, Measurements and Error Analysis’ though sounds preliminary, is not only important but has direct implications with all other chapters of Physics. Moreover, you will have at least some questions from this chapter whether in NEET, IIT JEE Main or JEE Advanced, AIPMT or in any other Medical and Engineering Entrance Exams or NTSE, KVPY etc.
Section wise notes (part-by-part from this & other chapters) containing important study materials (Tables, Formulae, etc.) have been/will be published in sequence.
Additionally, you have -
Solved 'India Study Solution Test Series’ each set containing exclusively selected 10 expected questions with hints & solutions from the chapter Units,Dimensions, Measurements and Error Analysis (click on the links given at the end of this post).
Also, scroll below to find a common syllabus for this chapter given at the end.
Physics and Physical World
The word 'Science' has come from a Lain word, 'Scientica' which means, 'to know'. Science is the subject of knowledge achieved by systematic approach through observation and experience, aimed at understanding natural phenomena in as much detail as possible.
A scientific approach (method) involves various interconnected steps -
Other Topics from this Chapter
|
Dimensions, Dimensional Formulae, Dimensional
Equation
|
Dimensional Analysis and its Applications
|
1. Systematic Observation
2. Controlled Experiment: A controlled experiment involves setting up two experimental conditions that are exactly same except for a single factor that the scientist manipulates.
3. Rationalisation or Qualitative and Quantitative Reasoning
4. Mathematical Modelling
5. Interpretation: Statement based on above observations and some scientific theory. It can be scientifically altered (falsifiable).
6. Verification or Falsification
Physics is one of the many disciplines of science originated from a Greek word ‘Fusis’, which means ‘nature’.
The two main thrusts in Physics are -
1. Unification: that is explaining diverse physical phenomena in terms of concepts and laws. For example, the same law of gravitation discovered by Newton also explains planetary motion, motion of moons around a planet as well as a body falling on the earth.
2. Reduction: It is deriving the properties of a bigger or more complex system from the properties and interaction of its constituent parts. For example, in thermodynamics the temperature is related to the average kinetic energy of molecules of the system.
The two main domains of interest in Physics are -
1. Macroscopic Domain: This includes Classical Physics which is study of objects of finite size that can be in a laboratory, in a terrestrial scale or even on astronomical scale. For example, mechanics, optics, electrodynamics and thermodynamics.
2. Microscopic Domain: It includes studies involving atomic, molecular and nuclear phenomena, also interaction of elementary particles like electron, protons.
Technology
|
Scientific Principle
|
Steam / Diesel / Petrol Engines:
|
Laws of Thermodynamics
|
Refrigerator:
|
Laws of Thermodynamics
|
Aeroplane:
|
Bernoulli’s Principle in Fluid Dynamics
|
Radio & Television:
|
Principle of Communication Systems using Electromagnetic Waves
|
Rocket Propulsion:
|
Newton’s Laws of Motion
|
Computer:
|
Digital Logic
|
Orbital Motion of Satellites:
|
Kepler’s Laws of Planetary Motion
|
Electron Microscope:
|
Wave Nature of Matter
|
Cyclotron:
|
Electromagnetic Force on Charged Particles
|
Nuclear Reactor:
|
Nuclear Fission
|
Electric Generator, Transformer:
|
Faraday’s Law of Electromagnetic Induction
|
Sonar
|
Reflection of Ultrasonic Waves
|
Pressure Cooker
|
Rising of Boiling Point of water by increasing pressure
|
Photocell
|
Photoelectric Effect
|
Post continues after the Ad -
Physical Quantities
1. Those quantities which can be measured by an instrument and by which we can describe the laws of physics are called physical quantities. For example - mass, length, time, velocity, force, density etc.
2. Measurement is necessary to determine magnitude of a physical quantity so that we can compare two similar physical quantities and also prove physical laws or equations.
3. A Physical Quantity is completely specified if, it has -
· Numerical value only (ratio): e.g. refractive index, dielectric constant etc.
· Magnitude only (scalar): e.g. mass, electric charge etc.
· Magnitude & Direction (vector): e.g. displacement, torque etc.
4. There are a few physical quantities which are not specified even by unit, magnitude or direction. Such physical quantities are known as Tensors for example, Moment of Inertia, Stress, Strain, Thermal Conductivity, Magnetic Susceptibility and Electrical Permittivity, etc.
5. Physical Quantity = Numerical Value (n) x Unit (u). Since the Physical Quantity will be a definite or constant value so, if the unit(u) changes, the magnitude(n) will also change but product 'nu' will remain same. That means magnitude of a physical quantity and units are inversely proportional to each other. Larger the unit, smaller will be the magnitude.
6. There are three types of Physical Quantities: (a) Fundamental or Basic Quantities. (b) Derived Quantities. (c) Supplementary Quantities.
Fundamental (Basic) Quantities:
There are a large number of physical quantities out of which only a few are elementary quantities. We define them as fundamental or basic quantities which are independent of all other quantities and cover the entire span of physics. All other quantities can be derived or expressed in terms of the fundamental quantities by multiplication or division. Fundamental quantities are total 7 in numbers: Length (L), Time (T), Mass (M), Temperature (K), Electric current (A), Luminous intensity (Cd), Amount of substance (mol).
Derived Quantities:
Physical quantities which can be derived from or expressed in terms of basic quantities are called derived quantities. For example - Momentum, Speed etc.
Supplementary Quantities:
Apart from 7 fundamental quantities, there are 2 supplementary quantities:
1. Plane angle (It is angle between two lines or the angle subtended by an arc of a circle at its center. Its SI unit is ‘Radian’).
2. Solid angle (Angle subtended by a given surface area of a spherical surface at its centre is called a solid angle. Its SI unit is ‘Steradian’).
Syllabus
Need for measurement of physical quantities, Units for measurement and Dimensions, System of Units - SI, Fundamental and Derived Units. Dimensional Formula and Dimensional Equations. Dimensional Analysis and its Applications. Significant figures and rounding off the numbers. Measurement of length, mass, and time. Accuracy, Precision of Instruments and error analysis.
Units, Dimensions, Measurements and Error Analysis - Solved Test Series, Practice Questions
- Physics MCQ Test Series with Solutions for NEET, IITJEE, BTech, MBBS and BDS Entrance Exams - Units, Dimensions, Measurements and Error Analysis
- Units, Dimensions, Measurements and Error Analysis - Physics MCQ test series and practice questions for BTech, MBBS, BDS admission tests
- Free online solved Test Series on Units, Dimensions, Measurements and Error Analysis - Physics questions answers for IITJEE, NEET