Chemistry >> States of Matter (Gaseous, Liquid, Solid)
Chemistry Guide: States of Matter - More Test Series with Solutions
Chemistry online test series with fully solved practice questions exclusively helpful for preparation of AIPMT, NEET, IITJEE, Medical and Engineering Entrance Exam, MBBS Admission Test.
Chemistry Test Series on States of Matter (Gaseous, Liquid, Solid): (NEET / JEE Syllabus)
Gaseous State: Intermolecular Forces; Thermal Energy; Intermolecular Forces vs Thermal Interactions; The Gaseous State; The gas Laws; Boyle’s Law; Charle’s Law; Gay Lussac’s Law; Avogadro’s Law; Ideal Gas Equation; Kinetic Molecular Theory of Gases; Dalton’s Law of Partial Pressure; Graham’s Law of Diffusion; Maxwell-Boltzmann Distribution of Molecular Speeds; Behaviour of Real Gases; Ideal Gas Equation; Kinetic Energy and Molecular Speeds; Deviation from Ideal behaviour; Equation of State for Real Gases (van der Waals’ Equation); Compressibility factor; Liquefaction of Gases; Critical Temperature.
Liquid State: Vapour Pressure; Surface Tension; Viscosity.
Solid State: Classification of Solids; Classification based on Nature of Intermolecular Forces; Isotropy and Anisotropy; Crystal Lattice; or Space Lattice; Properties of Solids; Unit Cell; Calculation of number of atoms in a unit cell; Crystal systems; Designation of Planes in Crystals - Miller Indices; Close Packed Structures and Types of Crystal; Crystallography and X-Ray diffraction; Calculation of Packing Efficiency in FCC, CCP, BCC and in simple Cubic Unit Cell; Imperfections or Defects in Solid State; Applications of p-Type, n-Type Semiconductors
Medical and Engineering Entrance Exam Preparation
Chemistry States of Matter (Gaseous, Liquid, Solid)
Chemistry MCQ Test Series – Set 2 (Q. No. 11-20)
Question 11: The vapour of phosphine gas at 270C and 3 bar pressure has density:
a. 4.09 g mL-1
b. 4.14 g L-1
c. 2.04 kg L-1
d. 2.04 g L-1
Question 12: Amorphous solids:
a. Possess sharp melting points
b. Undergo clean cleavage when cut with knife
c. Do not undergo clean cleavage when cut with knife
d. Possess orderly arrangement over long distances
Question 13: The average kinetic energy of a gas molecule is given by –
a. 3/2 RT
b. 3/2 KT
c. 2/3 RT
d. 2/3 KT
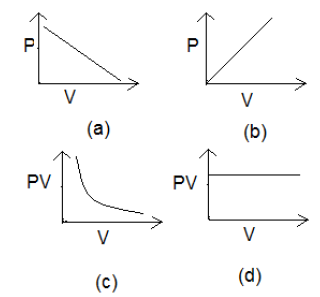
Question 14: Which of the following graphs correctly represents Boyle’s Law?
Question 15: By passing X-rays through copper sulphate crystals diffraction band is obtained. It was first observed by –
a. Max Van Laue
b. W. L Bragg
c. W. H. Bragg
d. W. L. Bragg & W. H. Bragg
Question 16: Percentage of free space in a body centred cubic unit cell is –
a. 32%
b. 34%
c. 28%
d. 30%
Question 17: Under similar conditions which of the following gases will diffuse four times as quickly as oxygen?
a. He
b. H2
c. N2
d. D2
Question 18: In stoichiometric defects, the ratio of positive and negative ions as indicated by chemical formula of the compound –
a. Decreases
b. Increases
c. Remains same
d. Cannot be predicted
Question 19: In a crystal both ions are missing from normal sites in equal number. This is an example of –
a. F – centres
b. Interstitial defect
c. Frenkel defect
d. Schottky defect
Question 20: Which set of conditions represents easiest way to liquefy a gas?
a. Low temperature and high pressure
b. High temperature and low pressure
c. Low temperature and low pressure
d. High temperature and high pressure
View / Download Answers [pdf]
Chemistry Guide: States of Matter - More Test Series with Solutions
Recent Posts
BIOLOGY
CHEMISTRY
PHYSICS